In-Vitro Diagnostic Device Regulation (IVDR) becomes effective May 26, 2022
Siemens Healthineers, a leading company in diagnostics, is strongly committed to a high-quality equipment and assay portfolio that enables healthcare providers to offer good patient care, medical diagnosis, and treatment monitoring.
Siemens Healthineers is on track to finalize IVDR certification and implement all requirements for more than 2700 products.
Siemens Healthineers proudly announces that we are one of the first companies to implement IVDR requirements in our comprehensive product portfolio, with the first IVDR certificate issued in July 2021.
We continue to pursue IVDR certification for all applicable Siemens Healthineers products within the extended transition phase mandated by the EU and thereby ensure continued availability of our vital in vitro diagnostic products.
The new European regulations for medical devices (EU MDR) and In-vitro Diagnostic Devices (EU IVDR), which will replace the current In-vitro Diagnostic Directive (IVDD) in May 2022, are not only a challenge, but a great opportunity to support our customers.
Siemens Healthineers has for years actively implemented EU regulations into its internal processes, and these upcoming changes will be no exception.
IVDR transition phase
We have been planning the transition to IVDR for our IVDD- and CE-marked products since 2017 and have dedicated significant resources and investment in preparing for these new regulations. We intend that our products will be fully compliant and IVDR-certified in time.
Our priorities are to satisfy both the regulatory requirements and our customers’ needs to ensure availability and continued supply of our products to healthcare professionals and patients.
We are pleased to confirm that our Notified Body for in-vitro medical Devices (IVDs) has been successfully designated under EU IVDR. This paves the way for us to obtain IVDR certification.
Update as of January 2022
European legislators published the amendment for a progressive rollout of the IVDR.
The application date of the IVDR is set for May 26, 2022. Originally, a very limited number of IVD products were allowed to benefit from a prolonged transition period until May 26, 2024. However, the unprecedented challenges of the pandemic have diverted resources from EU Member States, health institutions, and economic operators toward addressing the crisis, thereby hampering their capacity to comply on time with the changes introduced. In addition, there is a serious shortage of notified body capacity, making it impossible for many manufacturers to conduct the legally required conformity assessment procedures in time. Thus, January 28, 2022, EU legislators have decided to retain the application date of the IVDR—May 26, 2022—but allow for a more progressive rollout of the requirements for devices approved under the IVDD (“legacy devices”) to ensure a smooth transition to the new rules and ensure sufficient and timely supply of vital healthcare products in the EU.
The amendment does not change the requirements of IVDR but introduces prolonged transitional provisions for certain device classes to allow a progressive rollout of the regulation.
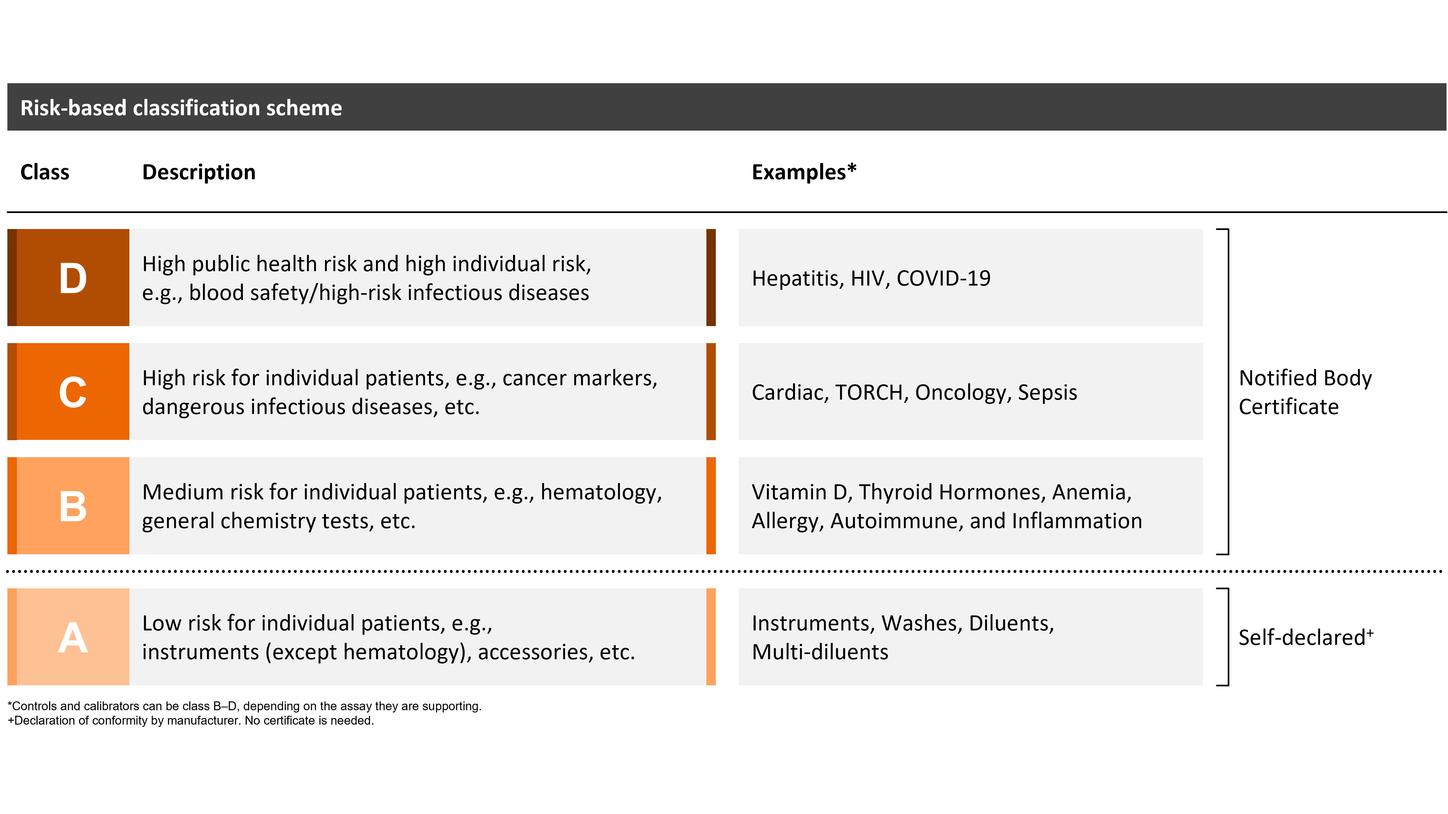
The length of the prolonged transition period depends on the device class (B–D), with transition periods extending until May 2025, 2026, or May 2027. Siemens Healthineers welcomes the decision of the EU legislators and considers the extended transition period a great opportunity to support customers in offering continuous patient care with high-quality diagnostic testing. However, certain provisions of the IVDR must be fulfilled from the date of application (registration, post-market surveillance, and vigilance requirements), and no significant change to the design or intended purpose is allowed.
Self-declared class A devices, as well as new and significantly changed IVDs, must comply with the IVDR starting on the date of application: May 26, 2022.
As a result, products certified under the previous directive and products certified under IVDR will coexist on the market during the transition period. Healthcare providers will benefit from the transitional provisions granted by EU legislators, which will help ensure continued availability of proven medical devices.
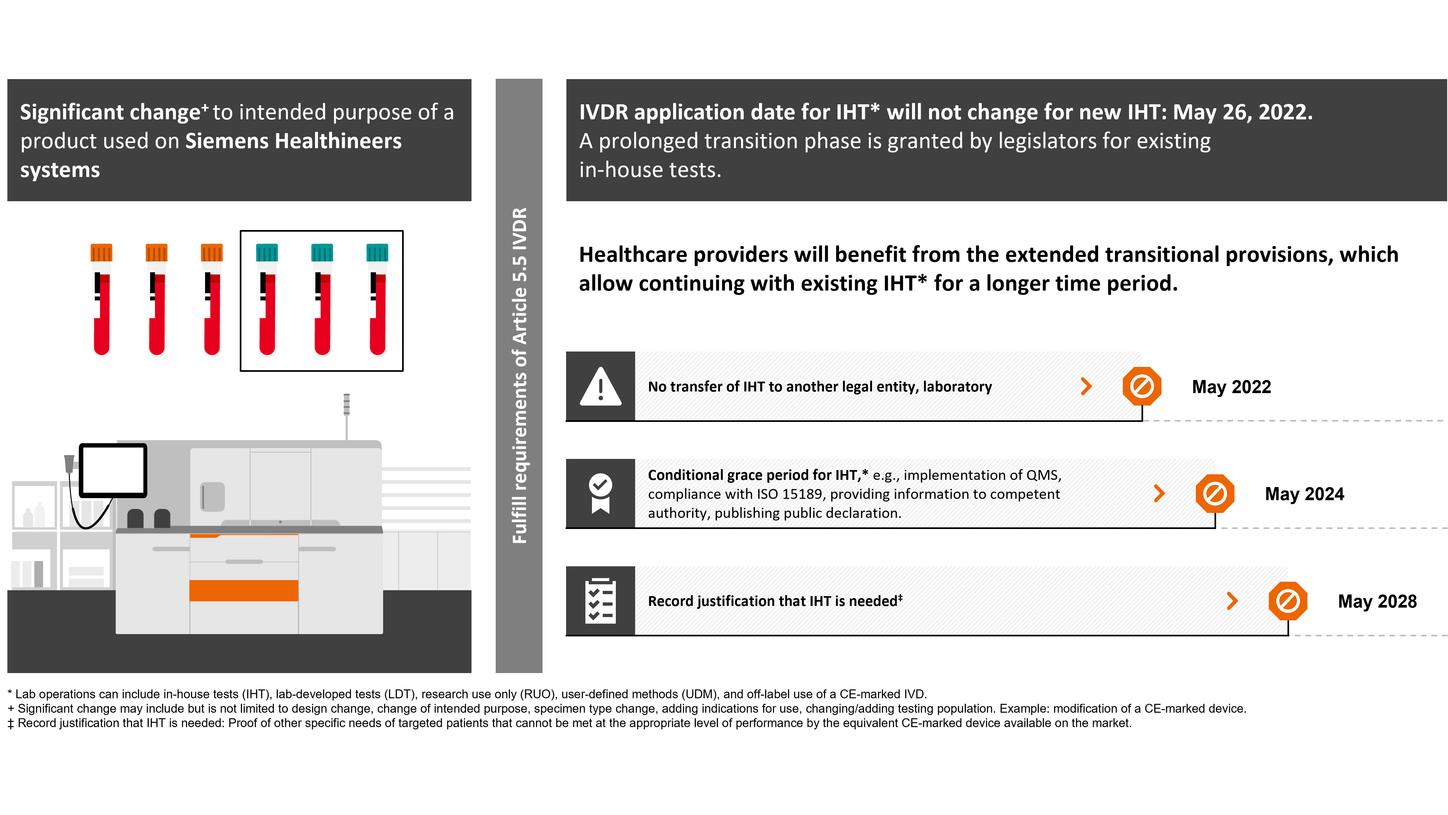
EU legislators also consented to deferred application of the requirements for devices manufactured and used within the same health institution, so-called “in-house devices,” also known as “user-defined methods” or ‘lab-developed tests”.
Healthcare providers and patient care will benefit from the additional transitional provisions granted by the legislators, which will facilitate the security of supply of vital IVD products.
More information will follow as part of the Siemens Healthineers IVDR series of communications or contact your local representative.
European legislators published the amendment for a progressive rollout of the IVDR.