AI-Rad CompanionProviding multi-modality imaging decision support
Did this information help you?
Please be advised that not all clinical extensions of AI RAD Companion: i) is not currently licensed for sale in Canada; and ii) may only be sold after a medical device license for the device has been issued by Health Canada in accordance with the Food and Drugs Act and Medical Devices Regulations. Safety and efficacy claims have not been reviewed by Health Canada and are subject to change. For greater certainty, the information provided with respect to the device does not constitute an offer to sell or to enter into a contract and cannot be accepted as such
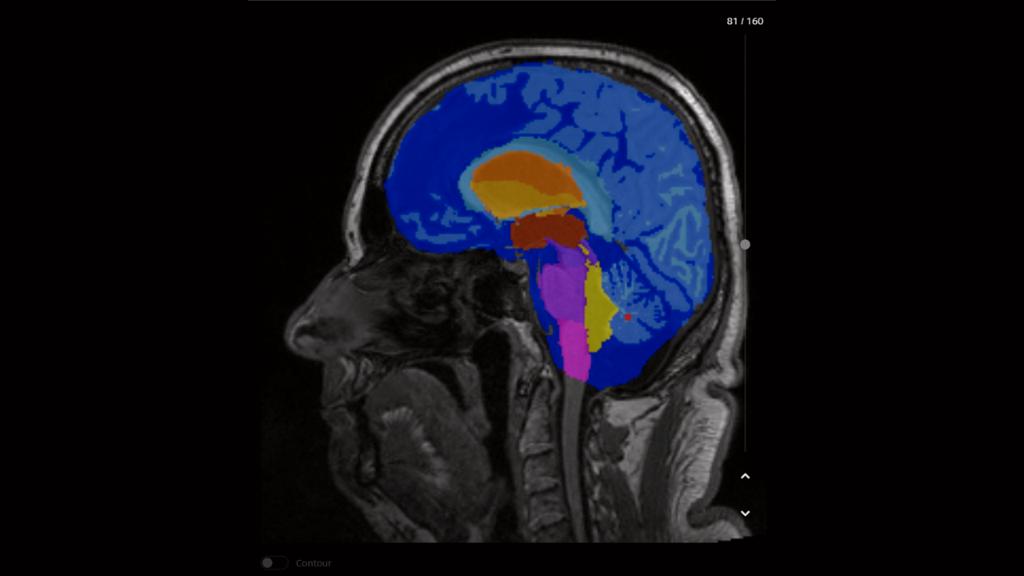
AI-Rad Companion Brain MR, Chest CT, Prostate MR and Organs RT are not yet commercially available in all countries and their future availability cannot be ensured.
Please be advised
that this device AI RAD Companion Chest x-Ray: i)
is not currently licensed for sale in Canada; and ii) may only be sold after a
medical device license for the device has been issued by Health Canada in
accordance with the Food and Drugs Act and Medical Devices Regulations. Safety
and efficacy claims have not been reviewed by Health Canada and are subject to
change. For greater certainty, the information provided with respect to the
device does not constitute an offer to sell or to enter into a contract and
cannot be accepted as such
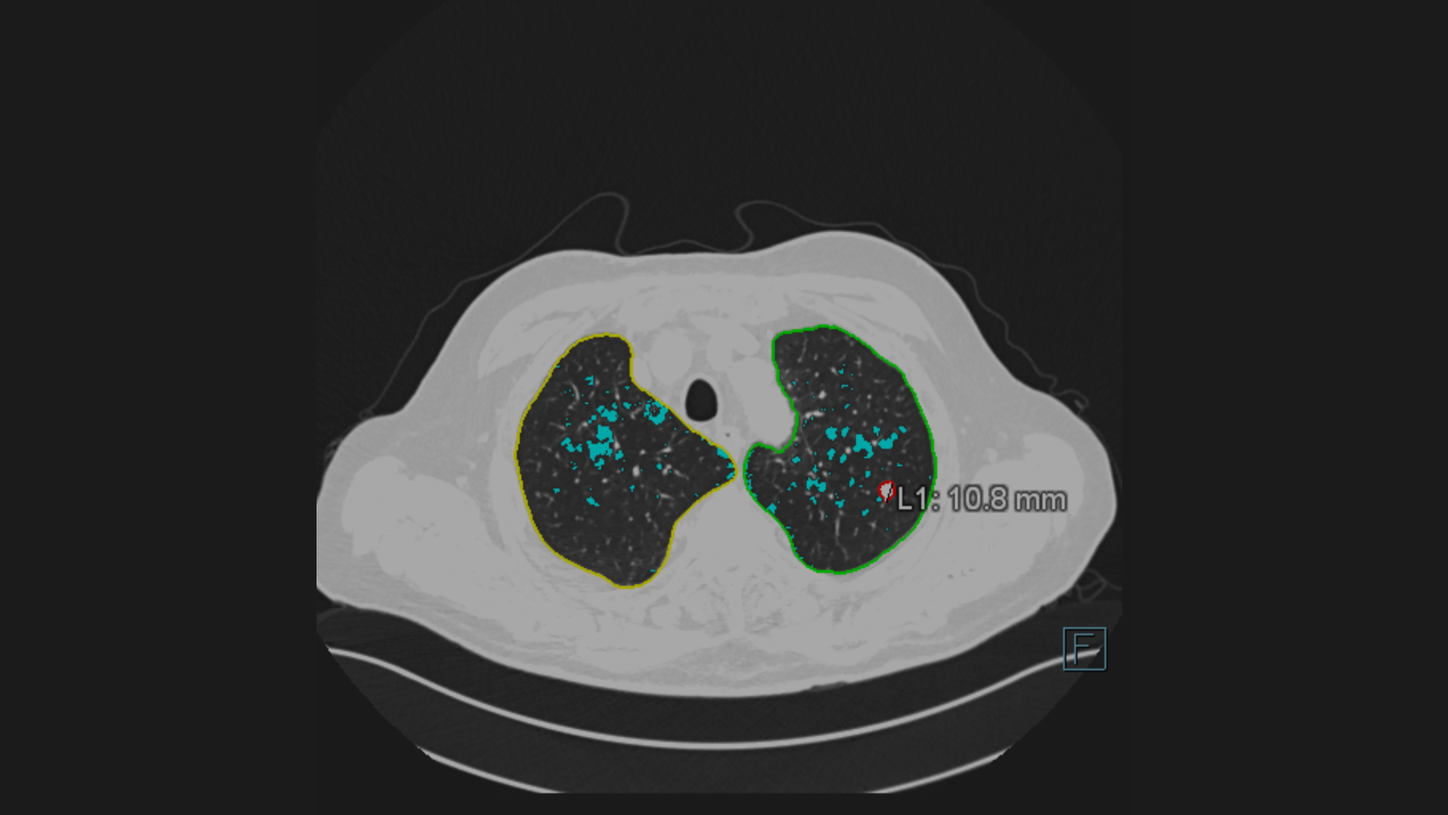
The pulmonary density functionality is not commercially available in all countries, and its future availability cannot be ensured. As soon as this functionality is available and cleared for your country it will become entitled to your institution.
The Pulmonary Density feature is new in VA12A without FDA Clearance. According to FDA policy “Enforcement Policy for Imaging Systems During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency” issued in April 2020, the manufacturer is allowed to market this feature without FDA-clearance. This policy is intended to remain in effect only for the duration of the public health emergency related to COVID-19 declared by the HHS, including any renewals made by the HHS Secretary in accordance with section 319(a)(2) of the Public Health Services Act (42 U.S.C. 247d(a)(2)). Pulmonary Density results are not indicated for the diagnosis of COVID-19. Only in vitro diagnostic testing is currently the definitive method to diagnose COVID-19.
For research purpose only. Not for clinical use. This prototype is still under development and not yet commercially available. Its future availability cannot be ensured.
https://pubs.rsna.org/doi/10.1148/radiol.2020201365