By Peter Bartenstein, MD
Data courtesy of Ludwig Maximillians University, Munich, Germany
History
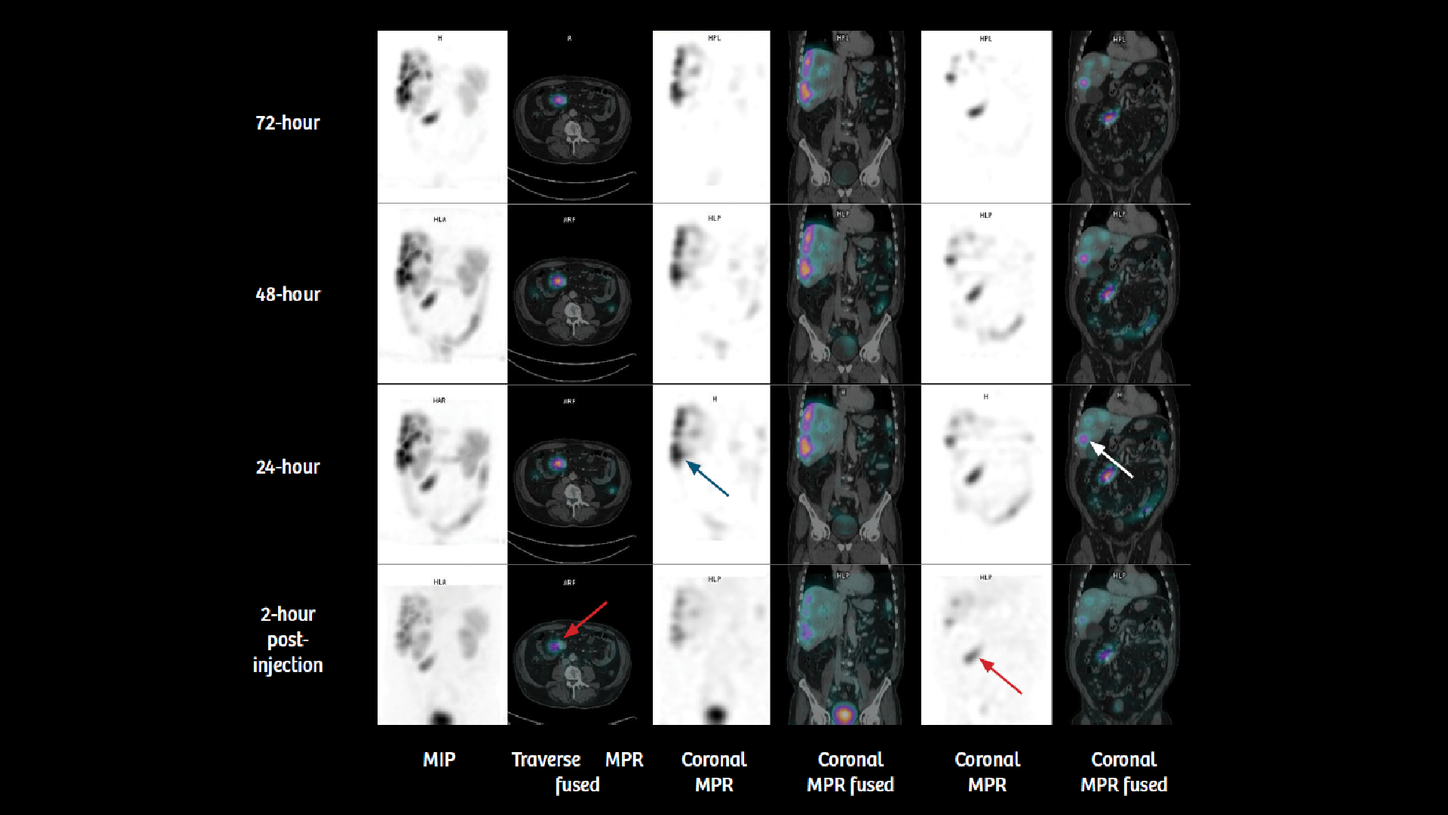
A 69-year-old male with intestinal carcinoid tumor with multiple liver metastases, confirmed using 68Ga-DOTATOC PET/CT imaging, was referred for radionuclide therapy with 177Lu DOTATATE. In view of the tracer-avid primary tumor and liver metastases demonstrating high uptake of somatostatin receptors seen on PET/CT, the patient was treated with 7.4 GBq of 177Lu DOTATATE as an intravenous infusion along with a standard regime of amino acid administration prior to and during therapy. Two hours following administration of the first therapeutic dose of 177Lu DOTATATE, the patient underwent a SPECT/CT study, which was followed by subsequent SPECT/CT studies performed 24, 48, and 72 hours after injection, in order to assess tracer concentration in the tumor and critical organs such as the kidneys, across 4 time points. The study was performed on a Symbia™ T16 SPECT/CT system, which had been calibrated using a NIST-traceable 75Se point source of known activity for accurate quantification of 177Lu radioactivity concentration. SUV measurements can subsequently be performed using quantitative SPECT/CT reconstructions of patient scans following 177Lu radiopharmaceutical administration.
SPECT acquisition was performed using 60 stops with 32 seconds per stop. The study was reconstructed using xSPECT™ ordered subset gradient conjugate method (OSCGM) reconstruction, incorporating attenuation and scatter correction. Volume of interest (VOI) was generated using fused SPECT/CT images over the tumor and critical organs, such as kidneys and liver, were evaluated to assess volumes and absolute tracer concentration within the VOIs as well as SUV.
Findings
Visual inspection of four sequential time point SPECT/CT scans (figures 1 and 2) demonstrates high tracer uptake in the primary intestinal tumor and multiple liver metastases with progressive increase in uptake by 24 hours. Very slow clearance is evident in substantial remaining tracer uptake by 72 hours. This suggests high initial uptake and slow washout in the tumor, which indicates the possibility of high tumor dose delivery due to increased tumor tracer retention time. The kidneys, however, show normal cortical uptake and progressive washout without pelvicalyceal tracer retention. Fast clearance suggests lower renal tracer retention time with possibility of low renal dose.
Quantitative SPECT/CT enabled SUV-based evaluation of changes in tracer concentration in the kidney and the tumor. It also allows generation of VOIs, including the tumor as well as critical organs, such as the kidney, liver, and spleen, in order to estimate absolute tracer concentration in the VOI in kBq/ml. Tumor, kidney, and liver volumes can be derived from VOI generated from fused SPECT/CT images with high accuracy. Tracer concentration from individual VOI could be derived from quantitative SPECT, leading to estimation of absolute tracer concentration within those VOI. Such estimations across 4 time points can be subsequently converted to the percentage of administered radioactivity concentration and fed into dosimetry software for calculation of effective dose to critical organs and the tumor.
The sequential quantitative SPECT/CT data and the estimated absolute tracer concentration and volumes of the left and right kidney, liver, spleen and two large liver metastases were processed by experimental dosimetry software to yield dose estimates. Four time-point studies were accurately registered to each other and VOIs generated and aligned across multiple time points to accurately assess quantitative changes in absolute tracer concentration across time and yield time activity curves.
It is clear from the dosimetry tables that the left and right renal dose (approximately 3.2 Gy each) is within expected levels, considering the initial therapeutic dose administered. The tumor dose in one large liver lesion is calculated to be 16.2 Gy, which suggests the possibility of significant cumulative dose to the tumor and favorable response. In view of the 23 Gy threshold of total renal dose across multiple therapy cycles generally accepted in radionuclide therapy1, the dose to both the kidneys calculated from this dosimetry evaluation following the initial therapy dose, suggests the possibility of two more therapies of similar doses of 177Lu DOTATATE without exceeding the threshold. However, in view of the tumor response to the initial therapy, subsequent therapies also require proper post-therapy imaging and dosimetry evaluation to ensure accurate calculation of the tumor and renal dose and adjustment of the amount and number of therapies, accordingly.
Comments
Most carcinoid tumors express somatostatin receptors, in particular SST2 receptors, which results in high uptake of radiolabeled somatostatin analogs, such as 177Lu DOTATATE, which is used for treatment of patients with advanced metastatic neuroendocrine tumors (NETs) with peptide receptor radionuclide therapy (PRRT). Apart from β emission, 177Lu DOTATATE is also a gamma emitter which allows planar and SPECT imaging to be performed following therapeutic dose, enabling demonstration of tumor uptake and estimation of tumor and critical organ dose along with prediction of therapeutic efficacy and need for and feasibility of multiple therapies. The standard therapy approach with 177Lu DOTATATE is for 4 cycles to be administered at 8-11 week intervals, with each therapy dose being 7-7.4 GBq. More cycles are added based on tumor and critical organ dosimetry and toxicity to bone marrow and kidney, evaluated by serial platelet counts and serum creatinine and creatinine clearance. Cumulative bone marrow and renal dose are the limiting factors concerning the number of therapy cycles and total dose delivered.
The objective is to give as many therapy doses as possible without crossing the cumulative renal dose limit in order to deliver the highest possible dose to the tumor without causing renal toxicity.Although concomitant amino acid infusion reduces the renal toxicity during PRRT, strict adherence to the practice of limiting cumulative renal dose to 23 Gy or less is currently the accepted approach to ensuring absence of significant renal toxicity during PRRT.1 In view of the potential impact of renal and bone marrow toxicity on the decision of the number of therapies of 177Lu PRRT, the accuracy of dosimetry for assessment of the radiation dose to the kidneys and bone marrow is critical to the therapeutic efficacy and outcome. Sequential whole-body planar scintigraphic acquisition following administration of therapeutic dose of 177Lu DOTATATE has been used for tumor and organ dose estimation by several sites. However, sequential SPECT/CT-based dose estimations have been proven to improve accuracy, especially for renal dose, since proper separation of the kidney from the liver and adjacent tumor, and abdominal uptake is often difficult using a planar study.2
Comparison of whole-body planar scintigraphy-based and sequential quantitative SPECT/CT-based dosimetry estimations in patients treated with 177Lu DOTATATE has shown that dosimetry using planar method leads to higher absorbed dose calculations in most patients.2
Quantitative SPECT/CT enables accurate assessment of absolute concentration of the tracer within tumor and critical organs, with potential improvement in equivalent dose calculation. In this example, sequential quantitative SPECT/CT studies enabled accurate estimation of the volume of the tumor and critical organs such as the kidney, along with the absolute tracer concentration and SUV within these volumes, which served as inputs for dosimetry calculations.
Nephrotoxicity with 177Lu DOTATATE therapy has been estimated to be low, with average annual decrease in creatinine clearance of 3%.3 Amino acid infusion during PRRT has been shown to further reduce renal toxicity. In a large series of 323 patients treated with PRRT, none of the patients had an annual decrease in renal function of more than 20%, with the mean radiation dose to the kidneys being 20.1 ± 4.9 Gy.4 In patients with large tumor burden with high receptor density, sequestration of the radiotracer within the tumor may lead to reduced bioavailability to kidneys and bone marrow, thereby further decreasing the absorbed dose to critical organs (i.e., tumor sink effect).5 Accurate calculation of renal dose using sequential quantitative SPECT/CT would potentially help in properly identifying the patients who would be eligible for a higher number of therapies without exceeding the renal dose threshold limits, thereby maximizing tumor response probability without undue risk of renal toxicity. Sandstrom et al1 performed renal dose estimation using sequential planar and SPECT/CT following 177Lu DOTATATE therapy on 200 patients with metastatic NET. Anterior and posterior planar and SPECT/CT studies were performed at 24, 96 and 168 hours after administration of a therapeutic dose of 7.4 GBq of 177Lu DOTATATE. Absorbed dose to kidneys ranged from 2-10 Gy per therapy cycle, which is comparable to the present study. Mean cumulative renal dose was approximately 9 Gy for all therapy cycles. Kidney dose decreased by a median of 39% between the first and third therapies. With a 23 Gy limit to the kidneys, 50% of the patients could receive more than four cycles of therapy. However, dosimetry studies concluded that 20% of patients could tolerate less than four therapy cycles without crossing renal dose limits. Such a possibility of undertreating patients based on dosimetric estimations of renal dose, the progressive lowering of renal dose in subsequent therapies and the overall low incidence of renal toxicity, opens up the possibility of improved selection of patients for further therapies based on more accurate dosimetry studies made possible by quantitative SPECT/CT.
Conclusion
Following therapeutic administration of 7.4 GBq of 177Lu DOTATATE in a neuroendocrine tumor patient with multiple functioning liver metastases, quantitative SPECT/CT was performed at 2, 24, and 78 hours. Data was used for determination of tumor and renal tracer concentration and volume across multiple time points in order to generate time activity curves and calculate tumor and renal dose. Calculated absorbed dose to the kidneys were within expected limits, enabling further radionuclide therapies to be delivered to this patient. High tumor dose calculated from dosimetry studies suggest the possibility of significant response following successive therapies.
Examination protocol
Scanner: Symbia T16