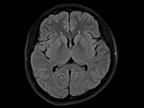
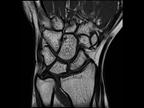
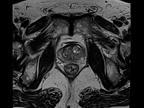
The healthcare market is undergoing a fundamental change. Healthcare providers have to ensure better outcomes for more patients at lower cost – despite shortage of skilled personnel.
Siemens Healthineers understands this challenge. The new 1.5 Tesla scanner MAGNETOM Sempra provides answers to these challenges – helping you achieve daily success with

MAGNETOM SempraDaily success with 1.5T.
1
Data on file; results may vary.
2
The MRI restrictions (if any) of the metal implant must be considered prior to patient undergoing MRI exam. MR imaging of patients with metallic implants brings specific risks. However, certain implants are approved by the governing regulatory bodies to be MR conditionally safe. For such implants, the previously mentioned warning may not be applicable. Please contact the implant manufacturer for the specific conditional information. The conditions for MR safety are the responsibility of the implant manufacturer, not of Siemens Healthineers.
3
The safety of imaging infants under two years of age has not been established. The responsible physician must evaluate the benefits of the MR examination compared to those of other imaging procedures.
4
Channels (coil elements) that can be connected simultaneously.
5
Minimum total space requirement for magnet, electronics, and console room.
6
SRS connection required, customer provides internet line to connect system to Siemens Healthineers infrastructure. Siemens Healthineers Connect Plan is subject to regional adaptions/restrictions.
7
Dane w dokumentacji; wyniki mogą się różnić
8
Wymagane jest połączenie ze zdalnymi usługami serwisowymi firmy Siemens, klient zapewnia łącze internetowe do podłączenia systemu do infrastruktury firmy Siemens. Pakiet usług serwisowych Siemens Healthineers Connect Plan podlega regionalnym dostosowaniom/ograniczeniom.
9
The MRI restrictions (if any) of the metal implant must be considered prior to patient undergoing MRI exam. MR imaging of patients with metallic implants brings specific risks. However, certain implants are approved by the governing regulatory bodies to be MR conditionally safe. For such implants, the previously mentioned warning may not be applicable. Please contact the implant manufacturer for the specific conditional information. The conditions for MR safety are the responsibility of the implant manufacturer, not of Siemens Healthineers.
10
The safety of imaging infants under two years of age has not been established. The responsible physician must evaluate the benefits of the MR examination compared to those of other imaging procedures.
11
Channels (coil elements) that can be connected simultaneously.
12
Minimum total space requirement for magnet, electronics, and console room.