By Partha Ghosh, MD and Michal Cachovan, PhD
Data courtesy of Royal North Shore Hospital, Sydney, Australia
History
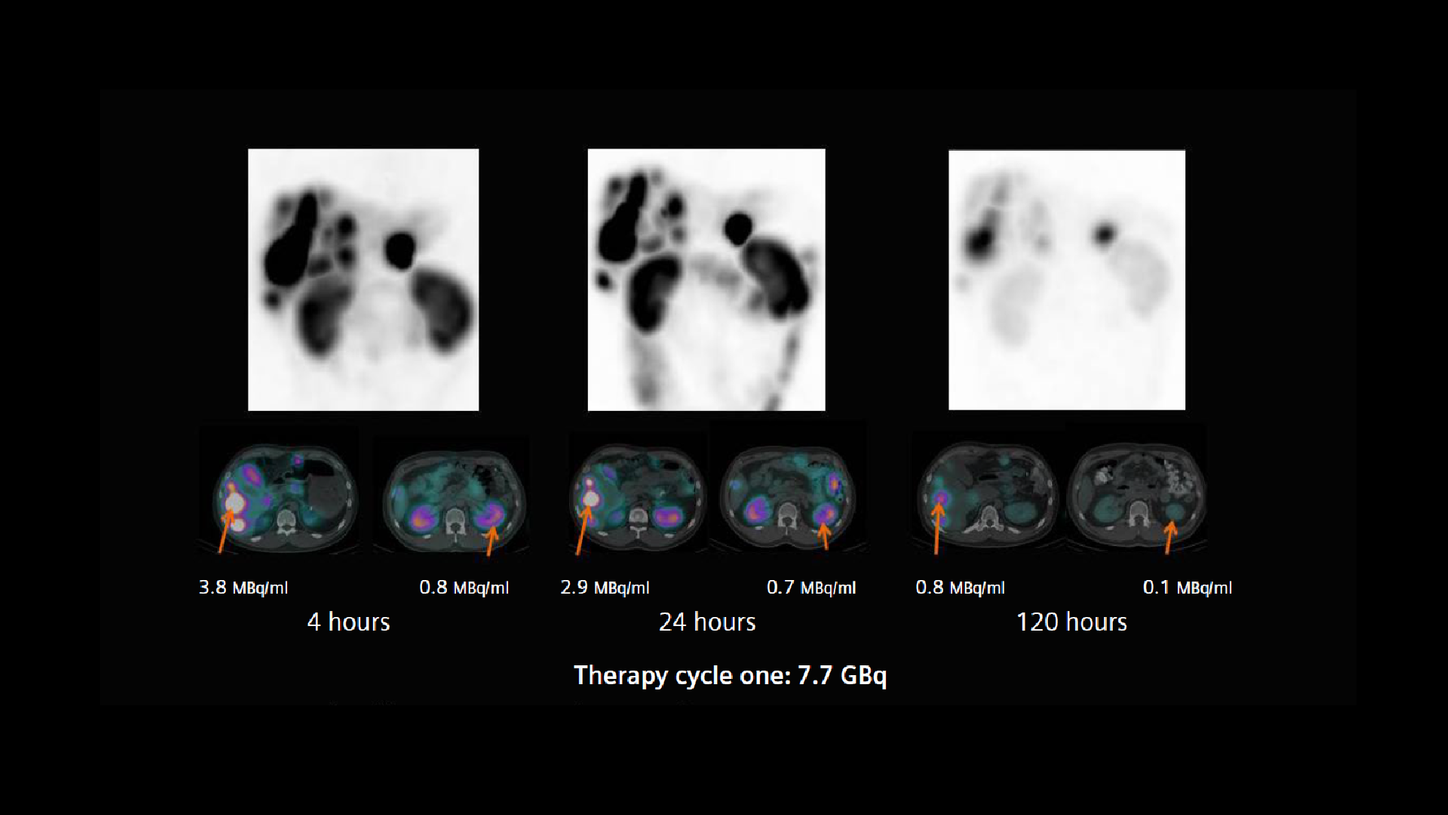
A patient with a neuroendocrine tumor and multiple functioning liver metastases, demonstrated by 68Ga DOTATATE PET/CT, was referred for 177Lu DOTATATE peptide receptor radionuclide therapy (PRRT). The first therapy dose of 7.7 GBq of 177Lu DOTATATE was administered in May of 2017. With an amino-acid infusion for renal protection started 30 minutes prior that continued until the completion of therapy infusion. Thirty minutes after the 177Lu DOTATATE infusion, the patient underwent an abdominal SPECT/CT study on a Symbia Intevo™ 6, utilizing xSPECT Quant™ technology. Following the initial low-dose CT acquisition (130 kV, 45 eff mAs, 6 x 1.0 mm collimation), a SPECT study was performed using 60 stops per detector with an acquisition time of 20 seconds per stop. xSPECT Quant reconstruction was performed using CT attenuation and scatter correction, which yielded quantitative data from which absolute tracer concentration in kBq/ml was derived.
Following the initial SPECT/CT, the patient underwent subsequent SPECT/CT studies using the same acquisition protocol at 4, 24, and 120 hours post infusion in order to generate sequential xSPECT Quant data.
The sequential xSPECT Quant datasets were evaluated on syngo®.via software in order to measure absolute tracer concentration within the tumor and critical organs, such as the renal cortex. The xSPECT Quant datasets were then loaded into Siemens Healthineers’ Dosimetry Research Tool (DRT).[a] The research dosimetry software aligns multi-timepoint SPECT/CT data and performs automatic organ segmentation based on CT and SPECT datasets to generate critical organ and tumor volumes of interest (VOI). The software also uses xSPECT Quant datasets with integrated absolute tracer concentration to generate voxel- and VOI-specific time-activity curves. With these time-activity curves, the software can perform 3D voxel-based dosimetry from the quantitative SPECT/CT data. Dose-volume histograms and absorbed-dose maps are also generated, which help assess the heterogeneity of dose distribution.
Findings
Visual and quantitative evaluations of the sequential xSPECT Quant datasets confirm the high uptake and slow clearance from the liver tumor, normal renal cortical retention, and fast washout. This suggests the possibility of a high tumor dose with normal levels of renal cortical absorbed-dose. Using the accurate quantitative data generated by xSPECT Quant as the basis, actual absorbed dose calculations were performed using the DRT.
From the dosimetry calculations (Figure 2), it is clear the renal dose is within the normal limits in spite of a high tumor dose. In view of the generally accepted threshold of 23 Gy as the maximum cumulative renal dose, a mean renal dose of 3.5 Gy supports the possibility of delivering at least 4 to 5 therapies of 177Lu DOTATATE dose. However, as tumor size and intensity of uptake often change following therapy, the renal dose delivered in subsequent therapies can easily be different from what is received during the initial therapy. As such, multi-time-point quantitative SPECT/CT imaging was performed in conjunction with subsequent therapies.
Eight weeks following completion of the first therapy, the patient was administered the second cycle of 177Lu DOTATATE therapy (July 2017). A dose of 7.4 GBq 177Lu DOTATATE was administered, as per standard protocol. xSPECT Quant acquisitions were performed 0.5, 4, 24, and 120 hours post-therapy administration, with acquisition and reconstruction protocols identical to the imaging performed following the first therapy. The DRT was used to generate absorbed dose values from the sequential xSPECT Quant data.
The second therapy cycle results in an increased uptake in the liver metastases with slow washout, as demonstrated by the retained tracer within metastatic lesions at 24 and 120 hours. The tracer concentration within the metastases is slightly lower after the first therapy cycle with a slight decrease in metastatic volume, which reflects a positive response to the first therapy. Bilateral renal-cortical uptake appears normal with slow initial washout and a significant amount of retained tracer at 24 hours but then subsequent fast clearance with minimal cortical retention at 120 hours.
The dosimetry research software generated absorbed-dose maps that show a calculated mean renal-cortical absorbed dose of 4.43 Gy for the right kidney and 4.84 Gy for the left kidney, which are considerably higher than that received by the kidneys following the first therapy. This may be due to the slightly lower level of tumor uptake and higher level of circulating tracer available for renal clearance, with consequent higher renal dose.
The tumor dose (for the same lesion in which dosimetry was performed after first therapy) shows significant reduction with mean absorbed dose of 4.3 Gy compared to mean dose of 15 Gy delivered after the first therapy. This reflects reduction in tumor size and number of functioning tumor cells following the first therapy.
The results for the second therapy cycle clearly indicate a slightly higher renal dose, but this was not considered excessive given the 7.4 GBq 177Lu DOTATATE administered. The tumor dose, however, was significantly lower, at least for the largest initial lesion studied. Given the level of tumor size reduction and intensity following the first therapy, second, and subsequent therapies are expected to deliver high tumor doses and further impact the tumor response.
The third therapy cycle administered 7.7 GBq of 177Lu DOTATATE in October 2017, 12 weeks after the second therapy. This cycle used identical protocols for therapy administration as well as sequential xSPECT Quant studies. The images acquired following the third therapy cycle (Figure 4) indicate substantially reduced size and uptake intensity in the liver metastases. There is fast washout of tracer from within the tumor with a low level of retained tracer at 120 hours. The renal cortical uptake is normal at 0.5 hours post therapy with fast clearance with negligible retention at 120 hours. The DRT calculates a mean renal dose of 4.3 Gy in the right kidney and 3.84 Gy in the left kidney, which are comparable to that received after the second therapy. The tumor dose is expectedly lower in the liver lesion that was considered for dosimetry in the first and second therapies, showing a much lower mean dose of 2.8 Gy with significant reduction in tumor volume.
As evident from the sequential images and absorbed-dose maps in Figure 4, there was further shrinkage in the liver metastases with significant decrease in intensity of uptake reflecting positive tumor response to radionuclide therapy. The right renal dose is comparable to that of the second therapy although the left renal cortical dose was significantly lower. Both renal doses correlate with expected results of normal cortical uptake and fast washout (as seen in the images).
Although the patient showed significant response of the functioning liver metastases with tumor shrinkage as well as notable reduction in intensity of tracer uptake, a fourth therapy cycle was administered in order to complete the therapy protocol and to deliver the maximum dose possible to the remaining functioning tumor tissue with negligible risk of exceeding critical organ toxicity thresholds as evident from the dosimetry calculations generated after the first three cycles.
The fourth therapy cycle of 7.05 GBq of 177Lu DOTATATE was administered in December 2017, 8 weeks following the third cycle. Sequential xSPECT Quant studies were acquired as per previous acquisitions. Reconstruction protocols and dosimetry were also performed using the DRT.
Sequential xSPECT Quant images following the fourth therapy cycle (Figure 5) show further decrease in size and uptake intensity of the liver metastases; reflecting additional response to radionuclide therapy. There is low tracer uptake within the liver lesions, which are much reduced in size compared to the previous study. Fast washout is reflected in low tumor absorbed dose calculations. The liver lesion considered for dosimetry in the previous therapy cycles shows a mean absorbed dose of only 2.1 Gy and a further reduction of size, thus reflecting impressive response to radionuclide therapy. The low uptake of the tracer, smaller lesion dimensions, and faster washout within the liver metastases as seen in sequential xSPECT Quant correlate well with the low tumorabsorbed dose calculated on the DRT. The absorbed dose to the renal cortices, calculated with the DRT, is comparable to the previous study and confirms normal initial uptake levels followed by fast washout.
In summary, from the MIP images (acquired 24 hours after administration of each therapy cycle) it is clear the metastatic lesions show a significant decrease in size and intensity of uptake, thus reflecting strong response to radionuclide therapies. At the time of third cycle, the largest metastatic lesion for which dosimetry was performed at each cycle has decreased substantially in size and intensity of uptake as evident from the representative images after each therapy cycle. Analyzing the mean tumor dose delivered to this lesion, it is apparent that there is a progressive decrease in mean dose delivered to the lesion which changes from 10.1 Gy after the first cycle to 4.3 Gy after second cycle to 2.1 Gy after the fourth cycle. This can be explained by a decrease in tumor size and intensity of uptake reflecting loss of functioning tumor tissue secondary to positive response to radionuclide therapy. The renal dose increases slightly after the second therapy reflecting increase in circulating tracer for clearance via kidneys secondary to decrease in tumor uptake following response to first therapy.
The DRT calculations indicate the cumulative dose to the left and right kidneys was 15.3 Gy and 16.18 Gy respectively, which is significantly lower than the 23 Gy limit for renal cortical dose in order to avoid renal toxicity.
Comments
This study shows excellent tumor response using four therapy cycles with overall low levels of cumulative renal cortical dose. This suggests the possibility of increasing administered dose for the initial therapies without risk of undue renal toxicity. Since tumor dose in subsequent therapies progressively decreases due to shrinkage and loss of functioning tumor cells with consequent lower tumor uptake in delayed therapy cycles, it is conceivable to administer higher initial doses during the first or second cycle in order to delivery higher tumor dose and obtain an earlier response.
xSPECT Quant enables accurate evaluation of absolute tracer concentration within tumor and critical organs across multiple time-point studies in order to compute tumor and renal total radioactivity concentration and residence times which enables accurate dosimetry. The DRT is capable of utilizing the quantitative SPECT/CT data to perform automated organ segmentation and 3D voxel based dosimetry to generate absorbed-dose maps and dose volume histograms in addition to generating mean-dose values from tumor and organ VOIs without a need for cumbersome dosimetry calculations. Seamless integration of quantitative SPECT/CT (xSPECT Quant) with 3D voxel-based dosimetry software produces absorbed-dose maps including dose color wash, which demonstrates not only the tumor and critical organ dose variations across multiple therapies but also the heterogeneity of the dose distribution within the same organ or tumor VOI. In tumors with heterogeneous distribution of functioning malignant cells, there is substantial heterogeneity of absorbed-dose and response within the same tumor may vary and this variability is well reflected on absorbed-dose maps obtained from voxel-based dosimetry.
In this case, the liver metastases all show high tracer uptake in the first study and substantial radiation dose was delivered to tumor, as evident from the dosimetry calculations and subsequent response levels. A similar study evaluated correlated cumulative tumor-absorbed dose and best response in 24 lesions treated with 2 to 6 cycles of 7.4 Gy 177Lu DOTATATE.1 The tumor response was in the range of 4.5-57% using RECIST criteria with the cumulative absorbed dose ranging between 20 and 340 Gy for tumors larger than 2.2 cm. The cumulative dose of at least 40 Gy was associated with best response and there was significant correlation between tumor- absorbed dose and overall response levels.
In the present study, absorbed dose was calculated using Siemens Healthineers’ Dosimetry Research Tool in which the largest lesion showed a mean absorbed dose of 10 Gy after the first therapy. The mean cumulative dose of 19.6 Gy in this lesion appears low when compared to the previously mentioned study. The lower level of mean absorbed dose to tumor in the second, third, and fourth studies, reflect a major decrease in tumor size and uptake secondary to response after the first therapy cycle. The initial absorbed dose to the tumor and the response following the initial therapy were the main drivers of tumor response in this particular patient.
Another study performed quantitative SPECT/CT at 0.5, 4, 24, and 96 hours after no carrier-added 177Lu DOTATATE administration in 13 patients with metastatic neuroendocrine tumors, each receiving three cycles with an average dose of 7.8 GBq per cycle.2 The mean renal dose of 3.1 +/- 1.0 Gy per cycle (range 1.1-5.4 Gy) or 0.40 +/- 0.13 mGy/MBq in this patient population is comparable to the mean renal-absorbed dose in this particular patient also from the same institution.
Evaluation of renal- and tumor-absorbed dose across multiple therapies of fixed dose 177Lu DOTATATE in a 200-patient study was performed using sequential SPECT/CT with the aim of optimizing the number of therapies based on cumulative renal- and bone marrow-absorbed dose estimation.3 Fixed dose (7.4 GBq/cycle) cycles were repeated until the absorbed dose to the kidneys reached 23 Gy unless therapy required to be stopped for other reasons. Almost half of the patients (49%) received 5-9 cycles (7.4 GBq each) in order to reach cumulative renal dose of 23 Gy. None of the patients reached the maximum bone marrow dose of 2 Gy. Out of all the patients, 23.5% had a partial response and 67.5% had stable disease. The objective tumor response was seen in 30.9% of patients, who reached a renal dose of 23 Gy and was also seen only in 13% of patients who did not reach 23 Gy.
It is evident from this study that substantial higher cumulative tumor dose can be achieved using either multiple fixed dose therapies or higher initial therapy doses without crossing renal thresholds, if accurate dosimetry is performed following every cycle and appropriate modifications to therapy approach are performed based on tumor and critical organ dosimetry.
Conclusion
Sequential xSPECT Quant and 3D voxel-based dosimetry, performed after each 177Lu DOTATATE therapy cycle, show normal renal-cortical dose with each therapy cycle and a tendency of slight increase during the late cycles, which reflects a reduction in tumor burden with each successive therapy cycle. The dose delivered to metastases also show a progressive decrease along with reduction in tumor burden. Four therapy cycles resulted in adequate tumor dose to achieve an impressive reduction in tumor burden. The present case example illustrates the reliable value of quantitative SPECT/CT (xSPECT Quant) and 3D voxel-based dosimetry tools for quantitative evaluation of tumor and renal uptake, response evaluation, and accurate estimation of absorbed dose across multiple therapy cycles in order to have a comprehensive understanding of overall patient response.