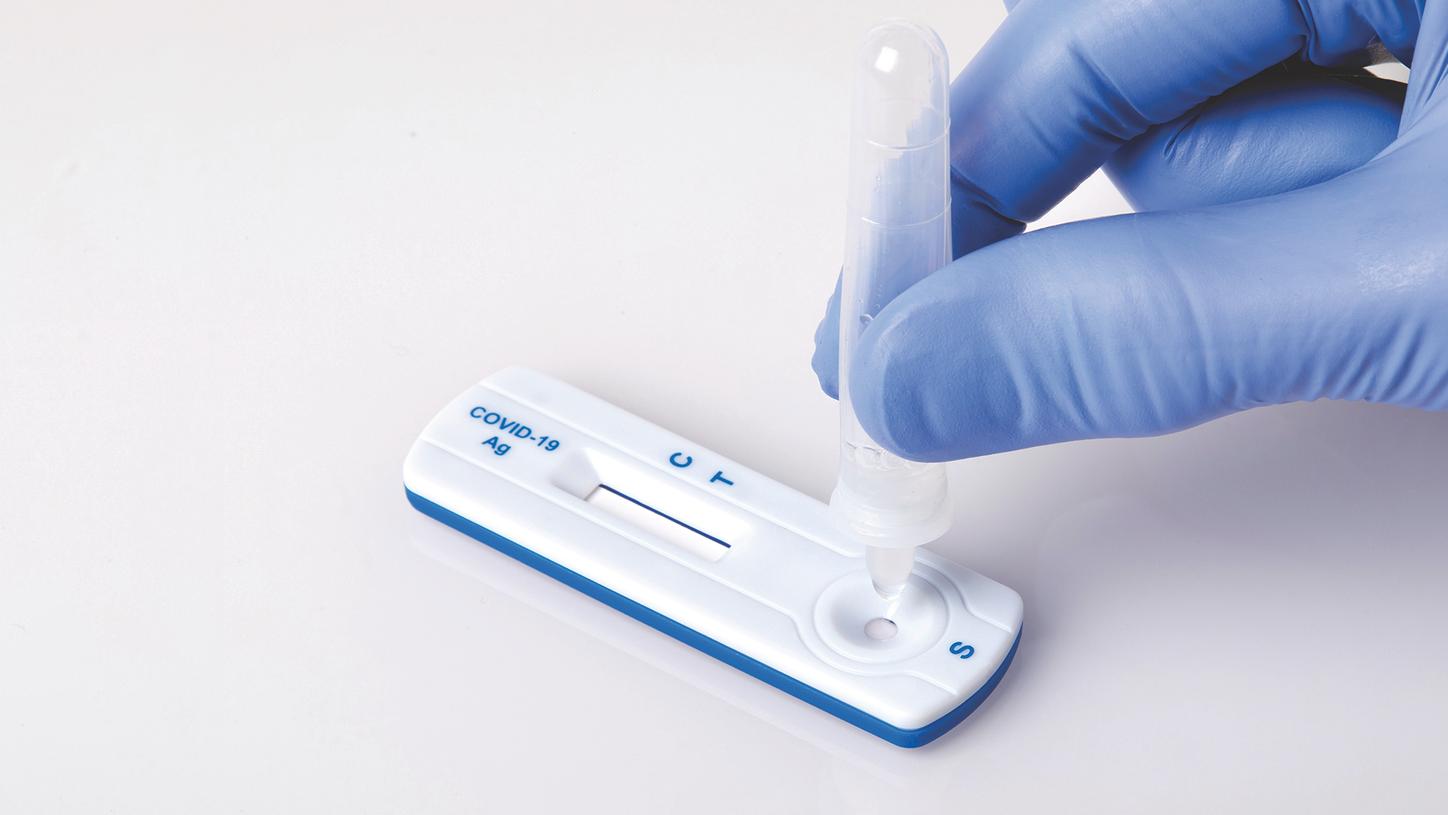
El camino hacia la reapertura seguraDesde pruebas de laboratorio a gran escala hasta pruebas en el punto de cuidado del paciente: múltiples opciones para COVID-19 de alta calidad nos llevarán a lo que está por venir.

¿Fue útil esta información?
*La disponibilidad de los productos y servicios varía en función del país y está sujeta a los distintos requerimientos regulatorios de cada uno y su disponibilidad futura no puede ser garantizada. En consecuencia, la presente tiene carácter meramente informativo y no constituye una oferta de naturaleza alguna. Por favor contacte a la oficina local de representación de Siemens Healthineers en caso de requerir mayor información.
1. CLINITEST - La prueba no precisa de profesionales de laboratorio especializados pero debe ser tomada y supervisada por un profesional de la salud.
2. El modo de uso de CLINITEST como auto-prueba o self test con la supervisión de un profesional de la salud está sujeto a las restricciones regulatorias de cada país . Por favor consulte con su representante local el modo de uso autorizado por las autoridades competentes.
Analytical time: time to generate a result on the cartridge or analytical device.
Distributed by Siemens Healthineers. CLINITEST Rapid COVID-19 Antigen Test: Not available for sale in the U.S. Product availability may vary by country.
CLINITEST - La prueba no precisa de profesionales de laboratorio especializados pero debe ser tomada y supervisada por un profesional de la salud.
El modo de uso de CLINITEST como auto-prueba o self test con la supervisión de un profesional de la salud está sujeto a las restricciones regulatorias de cada país . Por favor consulte con su representante local el modo de uso autorizado por las autoridades competentes.
This test has not been reviewed by the FDA. In the U.S., use of this test is limited to laboratories that are certified under Clinical Laboratory Improvement Amendments of 1988 (CLIA) to perform high-complexity testing.
Anterior nasal swab only available in the U.S. Product availability may vary by country and is subject to regulatory requirements.
Installed base of ADVIA Centaur XP, ADVIA Centaur XPT, and Atellica Solution analyzers.
IFCC interim guidelines on molecular testing of SARS-CoV-2 infection. https://doi.org/10.1515/cclm-2020-1412, September 18, 2020.
The SARS-CoV-2 molecular and antibody tests have not been FDA-cleared or approved. These tests have been authorized by FDA under an EUA for use by authorized laboratories. The molecular test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens. The antibody test has been authorized only for detecting the presence of antibodies against SARS-CoV-2, not for any other viruses or pathogens. These tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner. Product availability may vary from country to country and is subject to varying regulatory requirements.
Turnaround times are calculated based on theoretical analysis with one NUCLISENS EASYMAG and one Thermo Fisher ABI7500; not based on real workflow study. Using more instruments would decrease the total turnaround time.
FTD SARS-CoV-2 Assay (CE-IVD) Instructions for Use 11416283_en Rev. B, 2020-12 and FTD SARS-CoV-2 Assay (EUA) Instructions for Use 11416299_en Rev. C, 2021-01.
https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-reference-panel-comparative-data. Accessed on March 2, 2021.
Data on file at Fast Track Diagnostics, A Siemens Healthineers Company, Luxembourg.
Evaluation of sensitivity and specificity of four commercially available SARS-CoV-2 antibody immunoassays. Public Health England. 2020 Jul. GW-1386
Some claims are not available in all countries. The SARS-CoV-2 IgG assays are for semiquantitative use in the U.S. The semiquantitation claim for the SARS-CoV-2 Total assays is not available. The fingerstick claim is not available in the U.S. Claims for detection of neutralizing antibodies and correlation to PRNT are not available in the U.S. and are under development for the COV2T assays. Not available for sale in the U.S. Product availability may vary from country to country and is subject to varying regulatory requirements.
Installed base of ADVIA Centaur XP, ADVIA Centaur XPT, ADVIA Centaur CP, Atellica Solution, Dimension Vista®, and Dimension® EXL™ analyzers.
Lauring, AS and Hodcroft EB. Genetic Variants of SARS-CoV-2—What Do They Mean? JAMA February 9, 2021 Volume 325, Number 6. 529-531. doi:10.1001/jama.2020.27124
https://www.independent.co.uk/news/world/asia/wuhan-covid-variants-december-2019-who-investigation-b1802393.html. Accessed Feb. 15, 2021
Fontanet, A. et al. SARS-CoV-2 variants and ending the COVID-19 pandemic. www.thelancet.com Published online February 11, 2021 https://doi.org/10.1016/S0140-6736(21)00370-6.
JR Mascola et al. SARS-COV-2 Viral variants—Tackling a moving target. JAMA DOI: 10.1001/jama.2021.2088 (2021).
MMWR / January 22, 2021 / Vol. 70 / No. 3. 95-99. https://www.cdc.gov/mmwr/volumes/70/wr/pdfs/mm7003e2-H.pdf
Wu, K. et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv 2021.01.25.427948; doi: https://doi.org/10.1101/2021.01.25.427948
Xie, X., Liu, Y., Liu, J. et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med (2021). https://doi.org/10.1038/s41591-021-01270-4
Challen R, Brooks-Pollock E, Read J M, Dyson L, Tsaneva-Atanasova K, Danon L et al. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372(579). doi:10.1136/bmj.n579
Galloway SE et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage — United States, December 29, 2020–January 12, 2021. MMWR Morb Mortal Wkly Rep 2021;70:95–99. DOI: http://dx.doi.org/10.15585/mmwr.mm7003e2external icon.
Collier, D.A. et al. SARS-CoV-2 B.1.1.7 escape from mRNA vaccine-elicited neutralizing antibodies. medRxiv 2021.01.19.21249840; doi: https://doi.org/10.1101/2021.01.19.21249840
Wibmer C.K. et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. bioRxiv 2021.01.18.427166; doi: https://doi.org/10.1101/2021.01.18.427166
Johnson & Johnson announces single-shot Janssen covid-19 vaccine candidate met primary endpoints in interim analysis of its phase 3 ENSEMBLE trial. https://www.jnj.com/johnson-johnsonannounces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interimanalysis-of-its-phase-3-ensemble-trial.
Mahase, E. Covid-19: Where are we on vaccines and variants? BMJ 2021;372:n597 http://dx.doi.org/10.1136/bmj.n597
Karim SA et al. New SARS-CoV-2 Variants — Clinical, Public Health, and Vaccine Implications. NEJM. March 24, 2021. DOI: 10.1056/NEJMc2100362
Piccoli, L. et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology, Cell (2020), doi: https://doi.org/10.1016/j.cell.2020.09.037.
Premkumar et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 10.1126/sciimmunol.abc8413 (2020).
Wajnberg, A. et al., Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020 Dec 4;370(6521):1227-1230. doi: 10.1126/science.abd7728. Epub 2020 Oct 28. PMID: 33115920; PMCID: PMC7810037.
https://www.medrxiv.org/content/10.1101/2020.12.09.20245175v1 (same data from Pfizer FDA VRBPAC report)
Wibmer C.K. et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. bioRxiv 2021.01.18.427166; doi: https://doi.org/10.1101/2021.01.18.427166
Gundlapalli, A.V. et al. CDC COVID-19 Response, SARS-CoV-2 Serologic Assay Needs for the Next Phase of the US COVID-19 Pandemic Response, Open Forum Infectious Diseases, Volume 8, Issue 1, January 2021, ofaa555, https://doi.org/10.1093/ofid/ofaa555