History
A 60-year-old woman with adenosquamous cell carcinoma of the lung was treated with resection of the primary disease and adjuvant chemotherapy. Seven months following initial therapy, the patient developed recurrent disease in the form of brain metastases. She subsequently underwent resection of the metastatic cerebral lesions, but developed a second recurrence in the brain approximately seven months later. Spectroscopic MRI was performed, however the results were equivocal as radiation necrosis could not be definitively excluded. Dynamic PET imaging to obtain additional parametric indices of glucose uptake was acquired in order to further assess the lesion.
Findings
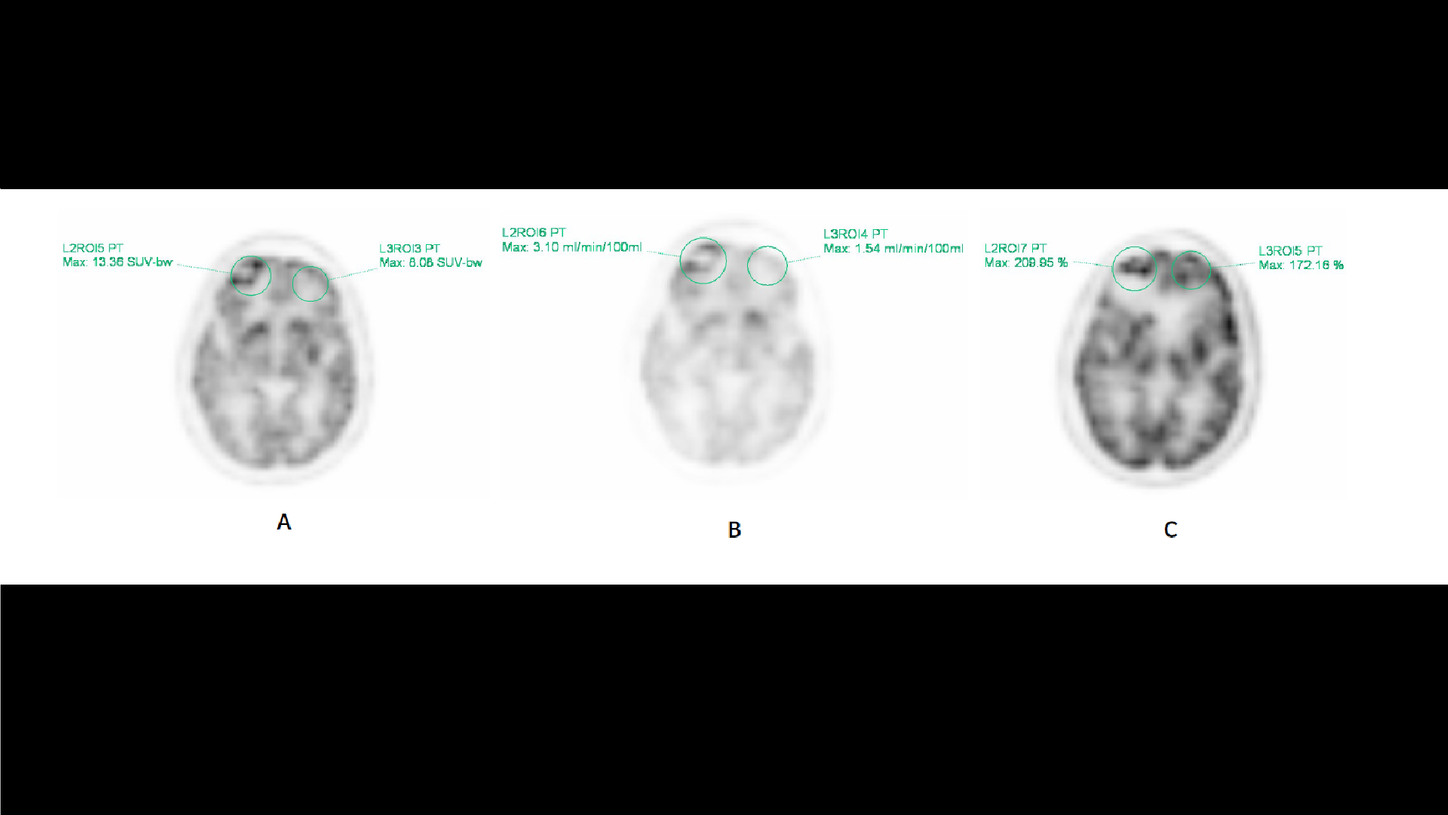
Dynamic whole-body PET imaging was performed with Siemens Healthineers’ FlowMotion™ Multiparametric PET Suite using the following imaging protocol. With the patient on the system table, 10.5 mCi (388.5 MBq) of Fludeoxyglucose F 18 injection (18F FDG)[a] was intravenously administered. This was immediately followed by a 6 minute, single bed, list-mode (LM) acquisition centered over the heart to obtain data used to generate an image-derived input function: a noninvasive alternative to arterial blood sampling. Wholebody scanning then commenced with continuous bed motion using FlowMotion, allowing data acquisition during both the forward and reverse passes of the gantry bed. A total of 19 whole-body passes were acquired: 4 passes at 2 minutes per pass and 15 passes at 5 minutes. Total imaging time was 89 minutes. Images from whole-body passes 14-19 corresponded to 60-90 minutes post-injection and were summed for the standard uptake value (SUV) and used for Patlak image reconstructions.
Standard SUV images demonstrated an area of discrete photopenia in the right frontal lobe with hypermetabolic activity along its periphery (Figure 1A). Patlak MRFDG (slope) images, reflecting tissues actively metabolizing 18F FDG, demonstrate the same intensely metabolically active, rim-enhancing photopenic lesion in the right frontal lobe (Figure 1B). Patlak distribution volume (DV or intercept) images, reflecting the ratio of free non-metabolized 18F FDG in tissue versus plasma, demonstrate increased activity in the right frontal lobe as well. However, the pattern of activity is markedly different from that seen on the SUV and MRFDG images as there are low levels along the periphery and high levels in the center of the lesion (Figure 1C).
Given the increased activity seen on the MRFDG images, which reflects tissue that is actively metabolizing 18F FDG, the findings are highly suggestive of residual disease in the right frontal lobe lesion along the periphery of a necrotic core. DV images, however, demonstrate activity within the center of this lesion, which has low MRFDG values. This is an interesting finding because it reflects the accumulation of free, non-metabolized tracer at the center of this lesion. Clinically, this could possibly reflect the physiological role of blood flow or blood pooling that occurs in the process of necrosis following therapy.
Given the equivocal MR results and the parametric PET findings that were highly suspicious for residual disease, a subsequent biopsy was performed, which did demonstrate disease.
Comments
18F FDG PET/CT is well established in oncology as a means to not only initially stage disease, but to also assess therapeutic response and evaluate recurrent disease. Despite its widespread use, its accuracy in performing the latter two tasks is somewhat limited. Metabolic activity secondary to posttherapeutic inflammatory changes can be misinterpreted as residual disease whereas the relative lack of activity in a mass can be erroneously interpreted as absence of residual disease. These limitations in metabolic PET/CT imaging are, in part, due to the methods of image acquisition. The origins of metabolic imaging were acquired dynamically, which allowed assessment of radiotracer kinetics from the time of injection. This type of dynamic acquisition and kinetic assessment translated to the direct measurement of 18F FDG metabolism in a volume of tissue (MRFDG).1 This method of imaging provided true quantitative data regarding physiologic and tumoral radiotracer activity. However, dynamic imaging proved time consuming, tedious, and uncomfortable for patients with its requirement for prolonged image acquisitions. Challenging postprocessing and mathematical analysis, as well as sampling of arterial blood via arterial line insertions to measure radiotracer concentrations in the blood over time posed additional challenges. As such, dynamic imaging was supplanted by the more convenient method of delayed post-injection imaging. This method of imaging allowed PET/CT to become more amenable to routine clinical use and dynamic imaging, from that time forward, was largely confined to investigational research studies.
In today’s practice, the acqusition of metabolic imaging follows a specified time delay after the intravenous injection of the radiotracer. Evaluation of physiologic and malignant tissue is via the SUV, a measure that quantitates radiotracer activity within a given area of tissue normalized to the distribution of the tracer throughout the body. The SUV, however, is only a rough estimate of 18F FDG metabolism in tissue because the components that comprise its measurement are variable (eg, the patient’s metabolic status at time of imaging and weight) and it does not factor in the plasma kinetics of a tracer that can markedly differ between patients.2 As such, the SUV is semi- quantitative and does not accurately characterize actual tumoral 18F FDG metabolism: a limitation that poses a challenge, particularly when it comes to characterizing a lesion following treatment. As illustrated in this case, the additional indices derived from parametric imaging allow a more accurate characterization of the right frontal lobe brain lesion. Although the lesion demonstrates prominent activity on the conventional SUV image, this activity could very well reflect post-procedural or post-surgical inflammatory changes and not disease. With the additional information provided by the MRFDG image (which reflects actual 18F FDG metabolism in a volume of tissue) it becomes clear that this lesion still bears residual disease along the periphery of a necrotic core. A subsequent biopsy confirmed the suspicion of disease.
Although post-therapy timing conventions help an interpreting physician discern whether activity seen on a post-therapy scan is inflammatory or actual disease (i.e. 10-14 days following chemotherapy, 6-8 weeks following radiotherapy), they are based more so on multiple anecdotal experiences as opposed to concrete data and conclusions from well-controlled clinical trials. Another factor to consider is the wide array of various chemotherapeutic agents and radiotherapy regimens. As it is likely that each affect cellular kill at different rates based on cancer type, histological grading, and intra-patient heterogeneity of lesions, it is also very likely that these loose guidelines either underestimate or overestimate the time needed for post-procedural inflammatory changes to resolve.
With the introduction of FlowMotion Multiparametric PET Suite, dynamic imaging and the additional measurements are now more convenient to incorporate into a clinical setting. This innovation addresses the challenges of dynamic PET imaging while providing true and reproducible quantitative data regarding physiologic and malignant tissue. It uses the wellestablished Patlak graphical analysis of a two-compartmental model for evaluation of radiotracer kinetics, allowing derivation of truly quantitative MRFDG and DV values. The convenience of FlowMotion allows data image acquisition in a full body sweep with both forward and backward movement of the table gantry. Also, the dedicated six-minute cardiac acquisition to indirectly measure activity within the arterial blood pool from automatically placed regions of interests (ROIs) over the aortic or left ventricle negates the need for painful, and often laborious, arterial blood line insertions. These innovations will not only make dynamic PET imaging considerably more convenient for clinical research applications, but will hopefully find its way back into routine clinical practice. As there is a plethora of literature3-7 demonstrating the clinical utility of parametric imaging in various cancer types, it is feasible that parametric imaging can be utilized for particularly challenging cases where additional kinetic information may be useful in reaching the final diagnosis.
Conclusion
As opposed to semi-quantitative SUV measures, 18F FDG PET/CT parametric imaging provides additional quantitative indices that allow for the measurement of radiotracer kinetics, which more accurately characterizes tumoral metabolic activity. As illustrated in this case study, parametric imaging can aid in the differentiation of post-procedural changes, which ultimately affects patient management.