A new PSMA-based approach that combines diagnosis and therapeutics could be a game-changer in the treatment of prostate cancer. We spoke to opinion leaders in nuclear medicine, urology, and biomedical engineering about how the new approach expands possibilities for patients and clinicians, as well as its implication for clinical management of prostate cancer.
Illustration by David Hänggi
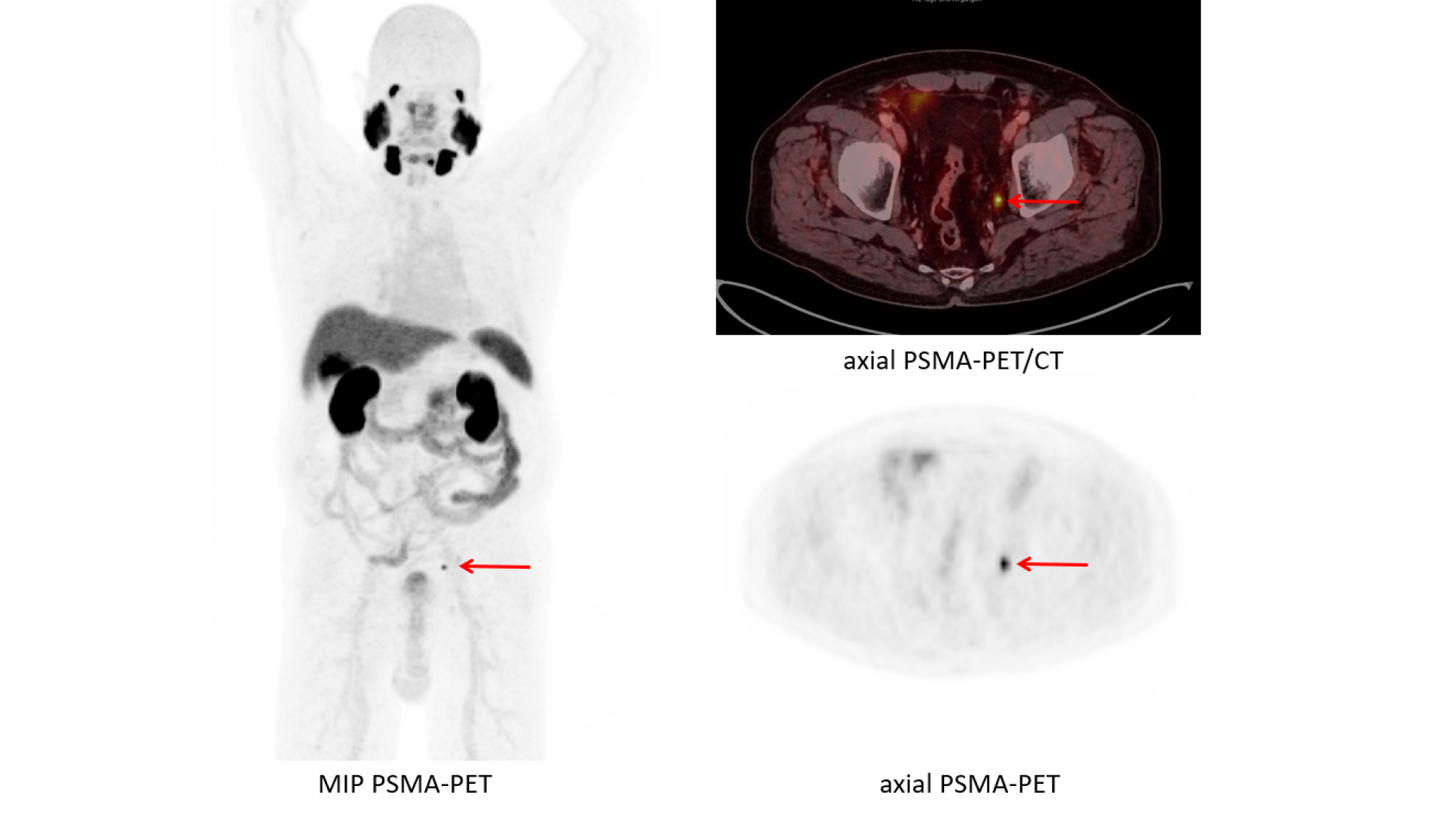
Data courtesy of Department of Nuclear Medicine, University Hospital Duesseldorf, Germany
Prostate cancer is still one of the most common and most lethal cancers in men. While the exact reasons and risk factors are still not fully known, therapy options have made significant progress in recent years.
A revolutionary new technique holds out the prospect of even greater progress in the future: prostate-specific membrane antigen (PSMA)-ligand analysis using a combination of innovative radiopharmaceuticals for positron emission tomography (PET) imaging. First clinical results are encouraging, and the United States Food and Drug Administration (FDA) recently gave its approval for multiple PSMA products. FDA approval and the broad availability of PSMA PET diagnostic agents in the United States that have been incorporated into the National Comprehensive Cancer Network guidelines1 will provide clinicians with options for improved understanding of the extent of disease—both at staging and in the case of a suspected recurrence of prostate cancer.
These developments provide the
green light needed to promote the
new diagnostic therapy for patients
with prostate cancer. Retrospective
and prospective PSMA studies confirm
the superior diagnostic accuracy as
well as the direct connection between
PSMA-derived tumor burden and
severity of disease.2-5 Many more prospective multi-center studies on this new method are still ongoing. Numerous experts, however, already see this combination of imaging and theranostics as offering potential in cancer care. Siemens Healthineers’ research and development efforts are being co-funded by the European Union’s Horizon 2020 research and innovation program.
Taking PSMA to the next level
The basis of this entirely new approach is PSMA. Similar to but not to be confused with PSA (prostatespecific antigen), PSMA is a small peptide that targets prostate cancer cells for different imaging agents in nuclear medicine, namely PET.
Now, however, teams of researchers
are taking PSMA imaging therapeutic
applications to the next level. In
several investigations in recent years,
they found that PSMA-targeting
molecules when combined with
Lutetium-177 isotopes are effective in
killing targeted cancer cells and tissue.6
Due to its high precision and efficacy, this method has the potential to
improve prostate cancer treatment in
later stages of the disease.
“These radioligands guide us with very exact information about if and where any tumor tissue is left.”
What is new about the PSMA PET
imaging that plays a decisive role
here? To answer this question, it is
essential to first look back in time.
The focus in the past 20 years was
on maximizing image resolution and
on high detection rates of tumors
and their structural changes, points
out Professor Frederik Giesel, MD,
MBA, an expert in nuclear medicine
and head of the department for
Nuclear Medicine at the University
Hospital Düsseldorf, Germany.
But this approach was akin to
“searching for the needle in the
haystack,” as Giesel puts it.
Therefore, instrumentation aside,
the specificity of a targeted peptide,
in the case of PSMA, opens the
door for a target treatment—
it moves PET squarely into
precision medicine.
Brian Helfand, MD, PhD, a urologist
based in Glenview, Illinois, USA, and
an expert on prostate cancer, agrees
with this position. “Prostate cancer
is not very straightforward for
clinicians. We need a lot of
information to help us decide on the
best treatment options that are
correct for every patient,” Helfand
says. Due to a lack of specificity and
reliability of conventional tests,
including PSA results, bone scan,
and CT scans, these medical
decisions were not as robust as they
needed to be. What is more, these
older imaging and lab results did not
always fit together coherently,
especially following seemingly
successful treatment or a period
with no tumor load, when one
indicator would turn positive but
the precise reason or location of
the recurrent tumor was unknown.
“But I am optimistic that this type of therapy, if successful, may even become an upfront therapy for many patients.”
Success in detecting earlier stage cancer tissues
The new radioligand therapies based on PSMA in combination with PET imaging are much more precise. “It feels like someone lit the Christmas tree,” says Giesel referring to the much more precise identification of new cancer tissue with these new diagnostic agents. And he adds, “These radioligands guide us with very exact information about if and where any tumor tissue is left.” This is a “major milestone” answering the previously unmet need of identification and treatment of prostate cancer recurrences, according to Giesel.
Just as with other radiotracer methods, PSMA is mainly used for already-diagnosed prostate cancers. “Currently, the diagnostic radiotracers are used in assessment of biochemical recurrence or high-risk primary prostate cancer where there is a significant chance of metastatic spread outside the pelvis,” says Bruce Spottiswoode, PhD, director and senior biomedical engineer at Siemens Healthineers.
“A common problem with many of our standard therapies for prostate cancer is serious secondary effects. The new therapy, however, might improve that to a large extent,” says Helfand. “It goes without saying that further research is still needed. But I am optimistic that this type of therapy, if successful, may even become an upfront therapy for many patients.”
PSMA PET/CT imaging plays a special role in this new approach. Selecting patients and monitoring their response to therapy is now increasingly more effective. But in addition to the technological and medical progress, close cooperation of all sides of healthcare plays a crucial role.
Considerations for implementation in clinical practice
An important issue is the ultimate implementation and application in clinical practice. “What is meaningful in terms of a workflow? How can we save time and minimize errors? What sorts of measurements can we automate to make things easier? And ultimately: What degree of cancer staging support should we offer to enable clinical decision making?” asks Spottiswoode. He emphasizes Siemens Healthineers’ close cooperation with many academic and clinical partners. “Not only to help us understand the bigger clinical picture, but also to improve the robustness of our research prototypes and to train our algorithms to recognize the subtleties of PSMA uptake.”
Helfand says that some open questions remain, such as how to identify prostate cancer in earlier or even non-aggressive stages. In these cases, the PSMA expression might be too low for therapeutic purposes. “Nevertheless, this technique has the potential to become an absolute game-changer for being able to diagnose and treat with two very similar molecules,” says Helfand. In an ideal future, a different set of radiotracers and theranostic agents might even be able to ultimately target all prostate cancer cells in the entire body. “There is a lot of potential to continue from here.” The first major milestone was reached with FDA approval of PSMA products. Further large-scale clinical studies on Lutetium-177 and PSMA PET scans are being conducted. If the initial results are confirmed—and everything points to that—a new chapter in prostate cancer therapy is about to begin. And in a mere five to ten years, the new theranostics approach for this disease could become the new normal.
“Not only to help us understand the bigger clinical picture, but also to improve the robustness of our research prototypes and to train our algorithms to recognize the subtleties of PSMA uptake.”
About the author
Florian Bayer is a journalist based in Vienna, Austria, who writes on healthcare topics. He worked as health editor at the newspaper Standard for four years